Japanese breakthrough will make wind power cheaper than nuclear
A surprising aerodynamic innovation in wind turbine design called the ‘wind lens’ could triple the output of a typical wind turbine, making it less costly than nuclear power.
science
/
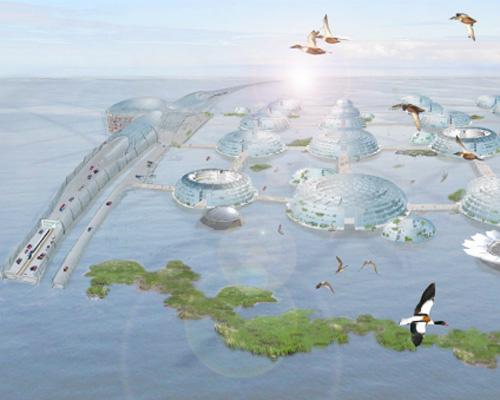
The Dutch are accustomed to building in flood-prone areas so perhaps it’s only natural for them to construct floating cities to cope with climate change. According to design company DeltaSync, such cities would be built to rise along with the sea level. Large blocks of polystyrene foam connected by frames of strong concrete would be used to float the dome-shaped buildings, and these structures would be linked via floating pedestrian bridges. Floating highways would even connect these aquatic cities, and heat drawn from the ocean’s surface would heat the city.
10 ways we could live on water
/
This is beautifully simple and complex all at the same time.
Fifteen uncoupled simple pendulums of monotonically increasing lengths dance together to produce visual traveling waves, standing waves, beating, and random motion.
The period of one complete cycle of the dance is 60 seconds. The length of the longest pendulum has been adjusted so that it executes 51 oscillations in this 60 second period. The length of each successive shorter pendulum is carefully adjusted so that it executes one additional oscillation in this period. Thus, the 15th pendulum (shortest) undergoes 65 oscillations.
More here
/
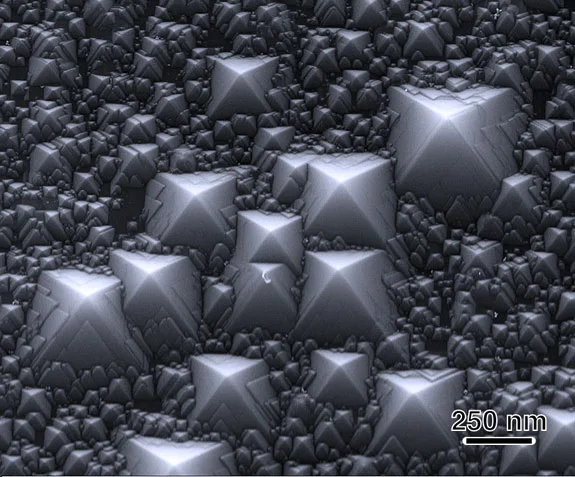
Chemical etching can spontaneously transform a flat silicon surface into one consisting of nanoscale facets, a process that covers the surface with tiny pyramids. Via.
/
hooray for smart people!
Student Invents Material With Highest Known Hydrogen Storage Capacity
A Ph.D. student at Rensselaer Polytechnic Institute has developed a new method for storing large amounts of hydrogen at room temperature using a version of the super-material graphene. Reportedly his material is inexpensive, easy to produce, and can store almost twice the amount of hydrogen than the U.S. Department of Energy’s ultimate target of 7.5% by weight at room temperature.
One of the biggest stumbling blocks to the widespread introduction of hydrogen-based vehicles is the fact that storing it with current technologies requires huge amounts of effort for little reward. Hydrogen itself is such a low-energy-density substance that you have to find ways to compress gigantic amounts of it into very small spaces to make it usable.
Up to now the available technologies were either putting it in a tank under very high pressure (read: explosive), or cooling it to incredibly low temperatures (like a couple hundred degrees celsius below freezing) and turning it into a liquid and then putting it in a tank (read: waste of energy). You might see how neither of these are really the optimal solution to storing hydrogen.
The holy grail of hydrogen storage would be a material that can collect huge amounts of it in a lightweight and compact form at room temperature—getting around the masses of energy needed to simply store it in the first place.
Well, now a bright student has figured out how to do just that—and then some. Javad Rafiee is a doctoral student in the Department of Mechanical, Aerospace, and Nuclear Engineering at Rensselaer. To come up with his solution, he used a combination of “mechanical grinding, plasma treatment, and annealing” to maximize graphene’s hydrogen storage capacity. The nanoscopic graphene molecules are arranged in a “chain-link” fence structure providing an extremely high surface area for the hydrogen to cling to. Couple that with graphene’s earth-shatteringly low density and you have the holy grail of hydrogen storage.
Javad recently won a $30,000 student prize for his work, but I’m thinking that’s just the beginning for this whiz kid. I’m sure there are plenty of other hurdles to overcome in this quest, such as how to get the hydrogen in and out of the structure quickly and how to scale it up and commercialize it, but, nonetheless, it’s exciting.
Source: EurekAlert!
/
un:
Check out “Number Gossip”
/
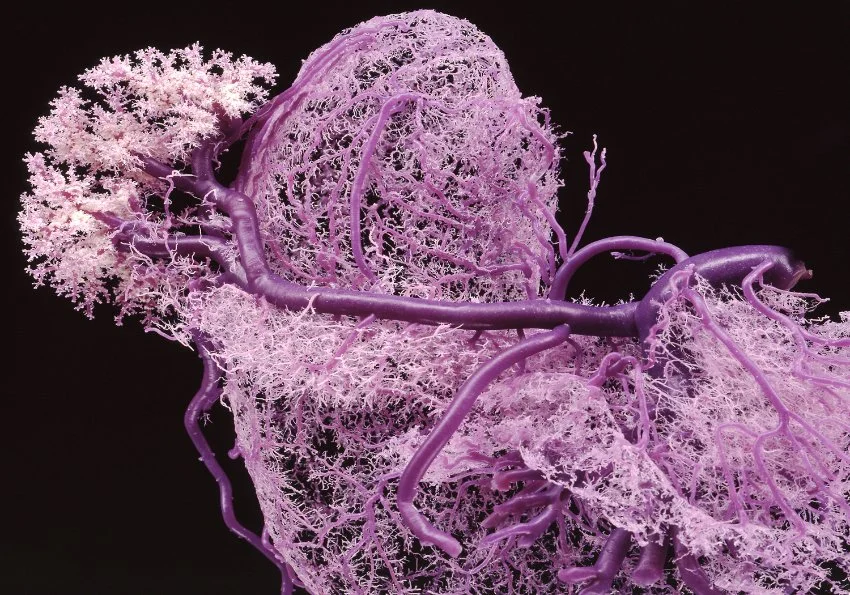
This series of veins have been plastinated using a method developed by Dr. Gunther von Hagens, the scientist behind the often controversial Body Worlds exhibits. A liquid plastic gets injected into the blood stream. When it hardens, scientists can study the system as it is in the human body.
This as a sculpture would be awesome.
/
(via quantumpossibility)
So pretty.
/
Theoretical flow of electrons in a two dimensional electron gas away from an electron source at the center. The same scattering that produces diffusion creates static branches of electron flow.Cover Article: Nature 410, 6825 (2001).
/
Blines and Circloids
Boids are elements that describe leaderless groups like flocks, herds or schools, according to the algorithm made in 1986 by Craig Reynolds. Boids have no mathematical equations describing their path: they move along individually, and as an emergent group steering clear of each other and moving in the direction everyone else is going.
Boids provide a good a way to describe natural compositions.
Below is an example of curves drawn with a combination of the Boids library and the Cornu spline library, a spiralling curve algorithm by Raph Levien.
Read more about Boids here:
http://nodebox.net/code/index.php/Boids
Or click through to see more pictures…
/
Laminar Flow http://en.wikipedia.org/wiki/Laminar_flow
/
Just your typical lipid structure… http://mhsp5lipids2.wikispaces.com/Structure